By Dr. Saunders at
Dry eye blepharitis syndrome: The simple role of biofilm in dry eye
New approach to dry eye
Standard dogma dictates that dry eye disease is quite complicated, with an overlapping disease presentation that stems from multiple etiologies. Based on this understanding, it would be heresy to suggest that dry eye is anywhere close to a simple process.
A new theory of dry eye has recently emerged, however, and is gaining traction among eye practitioners as well as opinion leaders in eye disease. In a 2016 paper in Clinical Ophthalmology, we proposed that blepharitis and dry eye are actually one simple condition: dry eye blepharitis syndrome (DEBS).1
If we take a step back from standard dogma and examine dry eye from a fresh perspective, we may uncover simple new treatments that provide long-anticipated relief for patients and practitioners alike.
What are biofilms?
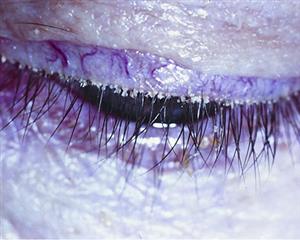
Understanding DEBS is easy if we first grasp the significance of a biofilm: a slimy, sticky film of bacteria that coats a surface.2
Bacteria are survivors, and they have done so for eons with their evolving survival skills. In nature, most bacteria exist not as free-floating individuals but rather as highly organized communities called biofilms. A biofilm is composed of a well-hydrated matrix of bacteria and their glycocalyx, a sugary coating that allows cells to adhere to and communicate with each other. This protective armor is highly difficult to penetrate–even by antibiotics, surgical iodine prep3 and, believe it or not, human white cells.
We propose that these bacterial survival skills are the very factors that cause dry eye disease.
Biofilms can form on any surface that provides moisture and nutrients. The eyelid margin—with its moisture, nutrients and warmth—is the perfect environment to cultivate a thriving bacterial biofilm. In fact, it would be unrealistic to suggest that a biofilm does not exist on the lid margin. A biofilm probably begins forming just after birth when the lids become colonized with bacteria.
The growth of the biofilm
A single bacterium has a low chance of survival. However, a biofilm containing billions of bacteria can easily survive. Within a biofilm, bacterial cells communicate with one another by secreting a chemical called homoserine lactone (HSL).4 The biofilm of a 2-year old child contains very low concentrations of HSL, thus the biofilm is non-pathogenic. The biofilm of a 50- or 60-year-old, however, has had decades to thicken, increase its bacteria load and produce high levels of HSL. This is a critical component of dry eye pathogenesis.
Once the bacterial colony senses that its numbers have reached a critical mass, as indicated by a high concentration of HSL, the bacteria use a process called quorum sensing to activate genes that elicit an inflammatory response in their human host.5 These genes encode virulence factors such as lipases, proteases, cytolytic toxins, scalded skin syndrome toxins and super-antigens (toxic shock syndrome) among others.6 Presumably, these virulence factors are tasked with liberating a larger food source for the burgeoning bacterial population.
Why does the biofilm wait to produce virulence factors once a critical mass has been reached? Because bacteria do not want to produce an inflammatory response from the host until they know they are abundant enough to withstand the host’s immune attack. So, the colony waits until they reach a quorum, indicating that they are safe within their thick biofilm armor.
Different strains, different styles
It is important to realize that not all strains of a species—such as the blepharitis-causing bacterium Staphylococcus aureus—are identical. Some strains of S. aureus are apt to create a biofilm that matures quickly and releases highly inflammatory toxins, which could produce severe blepharitis and chalazions in an 8-year-old. Other strains may produce thin, slow-growing biofilms that release relatively mild virulence factors over a person’s lifetime, and therefore spare an 80-year-old of any significant lid margin disease. It all depends on the strain of S. aureus.7,8
Is dry eye disease truly multifactorial?9 Many factors exacerbate dry eye (e.g, medications, hormonal state and reduced blinking), but the underlying etiology stems from a biofilm, present from infancy, that eventually achieves quorum-sensing gene activation and releases virulence factors.
What about the oft-noted “over-lapping presentations of different diseases,” which suggests that lacrimal dry eye disease is distinct from meibomian gland disease, which are both distinct from anterior blepharitis? Understanding how these different presentations relate to one another is easy, given the etiology of biofilm and an understanding of lid anatomy.
Stages of DEBS
Stage 1. Folliculitis: inflammation and edema of the lash follicles. This is always the first stage of disease, as it permits easy migration of the encroaching biofilm down the lash. Folliculitis eventually progresses from a small “volcano sign” to profound tissue edema around the lash. In severe cases, a sheathing of the lash with biofilm—often mistaken as cylindrical dandruff—can be observed once the lash grows out from the follicle.10 A thick biofilm attaches to and molds around the lash during dormancy, and then reveals itself once the lash leaves the follicle. Pull back the slit lamp and prepare to be amazed at how many patients have stage 1 DEBS, especially contact lens wearers.
Stage 2. Meibomian gland dysfunction (MGD): impaction and inflammation of the MG. Due to the MG’s size relative to the lash follicle and the small ductule with constant efflux of lipids, it simply takes longer for biofilm to accumulate and thicken within the MG. First, a simple plugging of the MG with meibofilm—a mixture of biofilm and meibum—reduces the quantity and quality of the meibum, sometimes referred to as non-obvious MGD with minimal inflammation. As the biofilm thickens within the gland, it eventually undergoes quorum-sensing and begins releasing virulence factors. This produces the inflammation referred to as posterior blepharitis. At this point, domes of meibofilm appear over each meibomian ductule.
It has long been thought that these little cream-colored domes over the MG were “caps” of keratin.11 But since the posterior lid margin consists of a non-keratinized stratified squamous epithelium, this explanation is highly unlikely. Within the context of biofilms, it is easy to understand the true source of these domes.
As the thickened meibofilm accumulates within the MG, the gland eventually reaches capacity. It may leak through the wall of the gland, causing a chalazion. The column of meibofilm gets forced up the ductule in an effort to escape the gland, but instead encounters a 40- to 50-year-old biofilm that has literally sealed off the orifice. The meibofilm bulges up against this original biofilm but cannot escape. Imagine covering the top of a toothpaste tube with a clear plastic wrap, then squeezing the tube. This is what happens to the MG when little domes of meibofilm become trapped atop each orifice. During MG probing, it is the penetration of this original biofilm that the practitioner may feel as the probe “pops” through.12
Stage 3. Lacrimilitis: impaction and inflammation of the accessory lacrimal glands of Krause and Wolfring. This always occurs after MGD. This is easy to understand if one reviews the lid anatomy and location of these tear glands. The glands of Wolfring are located along the top of the tarsal plate, and the glands of Krause are deep within the fornices.13 These 2 areas are quite distant from the margin, delaying access to a growing biofilm. However, biofilms can “seed” new areas by constantly dispersing tiny bits of biofilm into their environment (in this case, the tear film). If this happens hundreds of times a day, for 60 to 70 years, it is not inconceivable that a microscopic bit of biofilm eventually accesses these glands. It is also possible for biofilms to slowly creep up the inside of the palpebral conjunctive in an attenuated state (due to the constant blinking of the lids) and, after many years, reach the lacrimals by direct extension.
Could aqueous insufficiency underlie DEBS?
It may be difficult for some to accept that aqueous insufficiency shares a common etiology with MGD. However, many patients present with watery eyes, difficulty reading for long periods and a burning sensation. These patients are typically difficult to refract, as their vision changes with every blink. A low tear break-up time (TBUT), few meibomian puddles and occluded ductules confirm the diagnosis of evaporative dry eye disease. But patients may have difficulty understanding that diagnosis, given the tears running down their cheeks.
Patients with diseased MG and healthy lacrimal glands have the most common form of dry eye disease. However, patients often present with the exact opposite scenario: lots of lipids, perhaps a drop of oil running down the cheek, yet no aqueous. Except for Sjögren's autoimmune disease, which is an entirely separate pathology from lid margin disease, it is rare to encounter someone with a healthy MG and aqueous insufficiency.
This brings us to a key question: If aqueous insufficiency is truly a separate overlapping disease, wouldn’t we expect it to present prior to meibomian gland disease? At least once? But we never do, because it is simply a later stage of the same disease. It can’t happen because the morphology and anatomical location of the tear glands preclude it from happening. There is one common etiology: biofilm. It truly explains everything we see.
One might expect, after years of examining and unsuccessfully treating dry eye disease and blepharitis, that the biofilm theory would be a fait accompli! Unfortunately, decades-old dogma is difficult to dislodge. This new theory could bring relief to millions of patients, but it will likely take decades to be fully accepted. Meanwhile, few rigorous studies have been performed to support the current thinking.
An enlightened view of dry eye disease
Let’s take a step back, open our minds and lend credence to the simplest of explanations: Blepharitis and dry eye are actually one simple disease process.
In the field of dentistry, practitioners have achieved great success by simply educating patients on the importance of oral hygiene, plaque (biofilm) removal and the prevention of gingivitis and tooth loss. By educating patients about eyelid hygiene and prevention of blepharitis, the field of ophthalmology could enjoy similar success. A thorough and regular microblepharoexfoliation procedure could potentially bring relief to millions and forever change the way we treat this chronic debilitating disease. It really is all about the biofilm.
Author Disclosures
Dr. Rynerson is the President and CEO of BlephEx, LLC.
References
- Rynerson JM, Perry HD. DEBS – a unification theory for dry eye and blepharitis. Clin Ophthalmol. 2016; 10: 2455–2467. [PubMed]
- Lappin-Scott H, Burton S, Stoodley P. Revealing a world of biofilms – the pioneering research of Bill Costerton. Nat Rev Microbiol. 2014;12(11):781–787. [PubMed]
- Kıvanç SA, Kıvanç M, Bayramlar H. Microbiology of corneal wounds after cataract surgery: biofilm formation and antibiotic resistance patterns. J Wound Care. 2016 Jan;25(1):12, 14-9. [PubMed]
- Mireille Ayé A, Bonnin-Jusserand M, Brian-Jaisson F, Ortalo-Magné A, Culioli G, Koffi Nevry R, Rabah N, Blache Y, Molmeret M. Modulation of violacein production and phenotypes associated with biofilm by exogenous quorum sensing N-acylhomoserine lactones in the marine bacterium Pseudoalteromonas ulvae TC14. Microbiology. 2015 Oct; 161(10):2039-51. [PubMed]
- Wolf D, Rippa V, Mobarec JC, Sauer P, Adlung L, Kolb P, Bischofs IB. The quorum-sensing regulator ComA from Bacillus subtilis activates transcription using topologically distinct DNA motifs. Nucleic Acids Res. 2016 Mar 18; 44(5):2160-72. [PubMed]
- Knecht LD, O'Connor G, Mittal R, Liu XZ, Daftarian P, Deo SK, Daunert S. Serotonin Activates Bacterial Quorum Sensing and Enhances the Virulence of Pseudomonas aeruginosa in the Host. EBioMedicine. 2016 Jul; 9():161-169.[PubMed]
- Suzuki T, Kawamura Y, Uno T, Ohashi Y, Ezaki T. Prevalence of Staphylococcus epidermidis strains with biofilm-forming ability in isolates from conjunctiva and facial skin. Am J Ophthalmol. 2005 Nov; 140(5):844-850. [PubMed]
- Miyanaga Y. A new perspective in ocular infection and the role of antibiotics. Ophthalmologica. 1997; 211 Suppl 1():9-14. [PubMed]
- Bzdrenga J, Daudé D, Rémy B, et al. Biotechnological applications of quorum quenching enzymes. Chem Biol Interact. 2016 May 22; Epub. [PubMed]
- Gao YY, Di Pascuale MA, Li W, Liu DT, Baradaran-Rafii A, Elizondo A, Kawakita T, Raju VK, Tseng SC. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005 Sep; 46(9):3089-94. [PubMed]
- Uzunosmanoglu E, Mocan MC, Kocabeyoglu S, Karakaya J, Irkec M. Meibomian Gland Dysfunction in Patients Receiving Long-Term Glaucoma Medications. Cornea. 2016 Aug; 35(8):1112-6. [PubMed]
- Knop E, Korb DR, Blackie CA, Knop N. The lid margin is an underestimated structure for preservation of ocular surface health and development of dry eye disease. Dev Ophthalmol. 2010; 45():108-22. [PubMed]
- Stevenson W, Pugazhendhi S, Wang M. Is the main lacrimal gland indispensable? Contributions of the corneal and conjunctival epithelia. Surv Ophthalmol. 2016 Sep-Oct; 61(5):616-27. [PubMed]
By Dr. Saunders at
Surgical management strategies for trachomatous trichiasis
PLTR and BLTR: Two tried and tested techniques
Published in the August 2017 issue of Ophthalmology, the study Predictors of Trachomatous Trichiasis Surgery Outcome compares 2 tarsal rotation surgeries: posterior lamellar tarsal rotation (PLTR) and bilamellar tarsal rotation (BLTR).
Clinical trials have shown that PLTR and BLTR are more effective than alternative surgical procedures for the management of trachomatous trichiasis (TT) in trachoma-endemic settings. These tarsal rotation procedures are therefore recommended by the World Health Organization (WHO).
In particular, two trials examined the relative effectiveness of BLTR, tarsal advance and rotation, eversion splinting, tarsal advance (lid split), tarsal advance and grafting, and tarsal grooving. Both trials found the BLTR procedure to be superior than other strategies for the management of TT.1,2 Furthermore, BLTR can be readily taught to non-ophthalmologists, and can be easily and safely performed in rural health facilities. These are key advantages because, worldwide, most TT surgeries are delivered by non-physician health workers.
ALR: An unknown entity
To date, no randomized controlled trials have compared the anterior lamellar recession/repositioning (ALR) with either BLTR or PLTR for trachomatous trichiasis. One retrospective case series compared outcomes of BLTR and ALR procedures performed at different times. The study found a 46% risk of recurrance for BLTR and 17% risk for ALR, but these did not reach statistical significance.3 However, that study was limited by inconsistent follow-up times, a lack of surgeon standardization and an inadequate sample size, which make it difficult to draw meaningful conclusions.
A prospective noncomparative study conducted in Egypt reported a 34% recurrence rate at 6 months after ALR.4 A prospective non-comparative series from Iran of 32 cases with upper lid cicatricial entropion found a 25% recurrance rate at 1 year for ALR combined with blepharoplasty (excision of excess anterior lamella) and supratarsal fixation.5 These recurrence rates are higher than those reported in a recent trial comparing PLTR and BLTR (13% and 22%, respectively, at 1 year).6
In the aforementioned studies of ALR for TT, surgery was conducted by highly experienced surgeons; recurrence rates may be higher when performed by non-physician cadres with relatively limited training.7,8 Previous studies have consistently found that surgeon technique/ability is a crucial determinant of success.
Drawbacks of ALR
Critics have noted that ALR may not be sufficient for some phenotypes of TT because it does not address the posterior lamella. Therefore, it may prove ineffective in cases with severe posterior lamellar scarring and shortening, or cases with metaplastic lashes emanating from the posterior lamella, which occur commonly in trachoma.6,9-12 Because of this concern, ALR is usually reserved for cases with mild-to-moderate entropic trichiasis without metaplastic lashes emanating from the posterior lamella.
The literature generally advises that ALR be modified and combined with other more complicated techniques for various phenotypes of trachomatous trichiasis.9 For instance, in cases of thickened tarsus, which is usually the case in TT patients, ALR should be combined with a tarsal wedge resection. For cases with lid retraction secondary to the scarred, shortened posterior lamella, ALR should be combined with dissection of the levator aponeurosis and Muller’s muscle, followed by advancing the tarso-conjunctiva.9 This individualized and tailored surgical approach may be suitable for highly skilled oculoplastic surgeons, but is not a practical strategy for nurses who manage a high volume of cases in trachoma-endemic settings. It would be difficult to tailor surgical procedures to the various stages of disease, given the current TT backlog that must be rapidly addressed to prevent ongoing incident visual impairment. Fortunately, the Ophthalmology paper and other studies of BLTR and PLTR have shown that these procedures are overall quite effective 'one size fits all' approaches that can be safely and effectively performed by trained non-physicians.
While some physicians suggest refraining from making an incision in the posterior lamella, there is no evidence to support this stance. By contrast, there is strong evidence to indicate that surgical procedures involving the posterior lamella should be employe for severe cases of TT with metaplastic lashes and major lid shortening. Specifically, tarso-conjunctival rotation procedures, such as the PLTR, can provide adequate 180° rotation of the lid margin.9,13-15
Which surgery type is best?
A recent trial result comparing PLTR with BLTR shows that PLTR is more effective against the full spectrum of TT cases, with a lower rate of postoperative trichiasis among cases of varying severity. The findings suggest that PLTR is preferable for use in the programmatic management of varied phenotypes of TT, and where surgeries are performed by non-physician cadres with limited training.6 It is worth noting that TT recurrence, when encountered, is almost always minor (less than five lashes). This dramatically reduces the risk of corneal scarring and blindness from trichiasis.
In summary, the current literature indicates that tarsal rotation surgeries are superior to other approaches for the programmatic management of TT in trachoma-endemic settings. ALR has not been formally compared with tarsal rotation procedures, but is unlikely to be superior as it does not address the often-significant entropion caused by posterior lamella scarring.
References
- Reacher M, Huber M, Canagaratnam R, Alghassany A. A trial of surgery for trichiasis of the upper lid from trachoma. British Journal of Ophthalmology 1990; 74(2): 109-13.
- Reacher M, Muñoz B, Alghassany A, et al. A controlled trial of surgery for trachomatous trichiasis of the upper lid. Archives of Ophthalmology 1992; 110(5): 667-74.
- Barr K, Essex RW, Liu S, Henderson T. Comparison of trichiasis recurrence after primary bilamellar tarsal rotation or anterior lamellar repositioning surgery performed for trachoma. Clinical and Experimental Ophthalmology 2014; 42(4): 311-6.
- Ahmed RA, Abdelbaky SH. Short Term Outcome of Anterior Lamellar Reposition in Treating Trachomatous Trichiasis. Journal of Ophthalmology 2015; 2015: 568363.
- Aghai GH, Gordiz A, Falavarjani KG, Kashkouli MB. Anterior lamellar recession, blepharoplasty, and supratarsal fixation for cicatricial upper eyelid entropion without lagophthalmos. Eye 2016; 30(4): 627-31.
- Habtamu E, Wondie T, Aweke S, et al. Posterior lamellar versus bilamellar tarsal rotation surgery for trachomatous trichiasis in Ethiopia: a randomised controlled trial. The Lancet Global Health 2016; 4(3): e175-e84.
- Rajak SN, Collin JR, Burton MJ. Trachomatous trichiasis and its management in endemic countries. Survey of ophthalmology 2012; 57(2): 105-35.
- Burton MJ, Habtamu E, Ho D, Gower EW. Interventions for trachoma trichiasis. Cochrane Database of Systematic Reviews 2015; (11): CD004008.
- Kemp EG, Collin JR. Surgical management of upper lid entropion. The British journal of ophthalmology 1986; 70(8): 575-9.
- Rajak SN, Habtamu E, Weiss HA, et al. The Clinical Phenotype of Trachomatous Trichiasis in Ethiopia: Not All Trichiasis Is Due to Entropion. Investigative Ophthalmology & Visual Science 2011; 52(11): 7974-80.
- Rajak SN, Habtamu E, Weiss HA, et al. Absorbable versus silk sutures for surgical treatment of trachomatous trichiasis in Ethiopia: a randomised controlled trial. PLoS medicine 2011; 8(12): e1001137.
- Rajak SN, Habtamu E, Weiss HA, et al. Surgery Versus Epilation for the Treatment of Minor Trichiasis in Ethiopia: A Randomised Controlled Noninferiority Trial. PLoS Med 2011; 8(12): e1001136.
- Waddell K. A new clamp for bilamellar tarsal rotation for trachomatous trichiasis. Community Eye Health 2009; 22(69): 13.
- Kettesy A. ON GENESIS AND OPERATION OF THE CICATRICIAL (TRACHOMATOUS) ENTROPION OF THE UPPER LID. The British journal of ophthalmology 1948; 32(7): 419-23.
- Nasr AM. Eyelid complications in trachoma. I. Cicatricial entropion. Ophthalmic surgery 1989; 20(11): 800-7.
By Dr. Saunders at
Donor corneoscleral rim fungal cultures in the era of endothelial keratoplasty
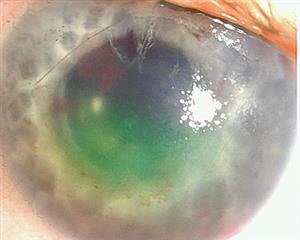
Postkeratoplasty infection is an infrequent but potentially blinding occurrence, with an incidence between 0.05% and 0.4%.1,2,3,4,5,6 When penetrating keratoplasty (PK) was the most commonly performed corneal transplant, the majority of postoperative infections were bacterial in origin.2 Although bacterial endophthalmitis is a devastating event, its rarity and poor concordance between donor rim culture and patient infection did not support routine culture of PK donor rims.7,8
However, the landscape of keratoplasty has changed over the past decade. In 2012, endothelial keratoplasty (EK) surpassed PK as the most frequently performed keratoplasty in the US. According to the EBAA statistical report,9 19,160 PK and 27,208 EK procedures were performed in the U.S. in 2015. Alongside this evolution in technique, an increase in case reports of fungal infections was noted, prompting large database studies that confirmed its rising prevalence.10,11,12 The question of whether or not to perform routine donor rim cultures is once again at the forefront and merits thoughtful review. The last Current Insight on this topic was published in 2008.8
Background
In 1990, the Eye Bank Association of America (EBAA) developed a system for transplanting surgeons to report adverse reactions possibly linked to donor tissus—such as primary graft failure and postoperative infection—to the source eye bank. All cases are then reported back to the EBAA, thereby producing a national donor cornea registry. Through this expansive data collection, an uptick in postkeratoplasty fungal infections was noted, and rates were found to be increasing over time.10,11
The surge was first noted in the early 2000s with PK cases,13,14 and it was unclear if it was related to a decrease in bacterial infections from the widespread adoption of Optisol-GS (Bausch and Lomb, Rochester, US).1 Soon after, Hassan et al1 found that storing PK donor tissue for 4 or more days was independently associated with a 3-fold increase in relative risk of fungal infection compared with the risk of bacterial infection in cases where Optisol-GS was used. More recently, Vislisel et al12 also found the median storage time of fungal culture-positive donor rims was 4 days.
The incidence of fungal infections has continued to grow in recent years, from 0.014% in 2013 to 0.023% in 2016,11 which coincided with the popularization of EK.9 These findings support that routine donor rim culture for fungus may play a more vital role in the age of EK.
Comparison of fungal infections after EK or PK
Aldave et al10 found that fungal infection was twice as likely to occur in patients undergoing EK when compared with PK, although this finding did not reach statistical significance. Similarly, the retrospective cohort study by Vislisel et al12 that assessed fungal keratoplasty infections after all types of keratoplasty found that all infections occurred after lamellar surgery: 3 of 4 cases of fungal keratitis occurred after EK, and 1 of 4 occurring after deep anterior lamellar keratoplasty (DALK).
The additional warming period required for EK processing has been purported to allow fungus to proliferate (Tu E. The effect of repeated warming cycles of corneal storage media on fungal infection risk in endothelial keratoplasty. EBAA/ Cornea Society Educational Symposium, November 13, 2015, Las Vegas, NV). Maintaining higher temperatures during storage is also preferred because the antibiotic activity of Optisol-GS at is improved in warmer environments.15 Furthermore, the lamellar interface may be more vulnerable to fungal growth due to its relatively hypoxic environment and sequestered nature that prevents normal immune mechanisms.16,17
Correlation of fungal culture-positive donor rims and clinical infection
In contrast to the discordance observed between bacterial culture-positive donor rims to clinical infection, multiple studies show fungal culture donor rims have predictive value.7,10-14,16 Keyhani et al14 studied 2,466 donor rims from 1998 to 2003, and found 28 (8.6%) were positive for fungus, specifically Candida species. All 4 patients who subsequently developed fungal endophthalmitis had positive donor rims of the same organism.
In 2007, a large meta-analysis by Wilhelmus et al7 showed a much higher agreement between rim culture and fungal infections when compared with bacterial infections. Most recently, Vislisel et al12 performed a study of 3,414 donor cornea rims and found 71 (2.1%) with fungal culture-positive donor rims. Four of the 71 patients (3 who underwent EK and 1 who underwent DALK) developed an infection (all Candida keratitis) requiring surgery. Several studies have found Candida species to be the most common causative agent in post-EK fungal keratitis and endophthalmitis.10,11,12,16
Aldave et al10 further examined corneas with culture-positive recipient fungal infection, and found that 73% (16 of 22) of the mate corneas were also positive. Fifteen of the 16 mate corneas were transplanted, and a subsequent infection developed in 10 of the 15 recipient eyes: endophthalmitis in 6 eyes, and keratitis in 4 eyes. This highlights the importance of sharing culture results with the source eye bank so that the mate tissue recipients may be more closely monitored. Furthermore, since fungal infections usually occur in a delayed manner, culture results may be available before clinical infection appears, and prophylactic antifungal therapy can be initiated.
Edelstein et al11 found the mean time to fungal endophthalmitis/keratitis from presentation was 33 days/45 days, whereas for bacterial endophthalmitis/keratitis, it was 2.5 days/6 days. Currently, there are no protocols to dictate the next steps when a positive fungal culture is discovered.
Conclusion
Although the incidence of postkeratoplasty fungal infections is low, it has been growing in recent years.11 The greater predictive value of fungal culture-positive donor rims and the high treatment burden of these cases16 support revisiting the role of routine culturing of donor rims, at least for fungus. Most recipients with fungal infections do not respond adequately to medical (topical and systemic antifungals) treatment and require surgical intervention to eradicate the infection,12 which may entail additional donor tissue as well as an increased risk of rejection. Discussion about the use of antifungal supplementation to cold storage media traces back to the PK era, but has now become of paramount interest in the age of EK. What effect antifungal supplementation has on donor rim cultures remains to be seen. In the meantime, it may be time to reconsider routine donor rim culture for fungus.
References
- Hassan SS, Wilhelmus KR, Medical review subcommittee of the Eye Bank Association of America. Eye-banking risk factors for fungal endophthalmitis compared with bacterial endophthalmitis after corneal transplantation. Am J Ophthalmol 2005;139(4):685-690.
- Kloess P, Stulting R, Waring G. Bacterial and fungal endophthalmitis after penetrating keratoplasty. Am J Ophthalmol 1993;115:309–316.
- Pardos GJ and Gallagher MA. Microbial contamination of donor eyes. Arch Ophthalmol 1982;100:1611–1613.
- Leveille A, McMullin F, Cavanaugh H. Endophthalmitis following penetrating keratoplasty. Ophthalmology 1983;90:38–39.
- Everts RJ, Fowler WC, Chang DH, et al. Corneoscleral rim cultures: lack of utility and implications for clinical decision-making and infection prevention in the care of patients undergoing corneal transplantation. Cornea 2001;20:586–589.
- Wiffen SJ, Weston BC, Maguire LJ, et al. The value of routine corneal rim cultures in penetrating keratoplasty. Arch Ophthalmol 1997;115:719–724.
- Wilhelmus KR and Hassan SS. The prognostic role of donor corneoscleral rim cultures in corneal transplantation. Ophthalmology 2007;114(3)440-445.
- American Academy of Ophthalmology. Current Insight 2008. Koreishi AF and Awdeh RM. Are donor corneoscleral rim cultures in penetrating keratoplasty clinically relevant? https://www.aao.org/current-insight/are-donor-corneoscleral-rim-cultures-in-penetratin. Accessed August 29, 2017.
- Eye Bank Association of America. 2015 Eye Banking Statistical Report. Eye Bank Association of America; 2016.
- Aldave AJ, DeMatteo J, Glasser DB, et al. Report of the Eye Bank Association of America medical advisory board subcommittee on fungal infection after corneal transplantation. Cornea 2013;32(2):149-154.
- Edelstein SL, DeMatteo J, Stoeger CG, et al. Report of the Eye Bank Association of America medical review subcommittee on adverse reactions reported from 2007 to 2014. Cornea 2016;35(7):917-926.
- Vislisel JM, Goins KM, Wagoner MD, et al. Incidence and outcomes of positive donor corneoscleral rim fungal cultures after keratoplasty. Ophthalmology 2017;124:36-42.
- Merchant A, Zacks CM, Wilhelmus K, et al. Candidal endophthalmitis after keratoplasty. Cornea 2001;20(2):226-229.
- Keyhani K, Seedor JA, Shah MK, et al. The incidence of fungal keratitis and endophthalmitis following penetrating keratoplasty. Cornea 2005;24(3):288-291.
- Kapur R, Tu EY, Pendland SL, et al. The effect of temperature on the antimicrobial activity of Optisol-GS. Cornea 2006;25:319-324.
- Tsui E, Fogel E, Hansen K, et al. Candida interface infections after descemet stripping automated endothelial keratoplasty. Cornea 2016;35:456–464.
- Grahl N, Shepardson KM, Chung D, et al. Hypoxia and fungal pathogenesis: to air or not to air? Eukaryot Cell 2012;11:560–570.